Inorganic and Organic Carbon, Nutrient, and Oxygen Data from the R/V Ronald H. Brown Repeat Hydrography Cruise in the Atlantic Ocean: CLIVAR CO2 Section A16N_2003a (4 June-11 August, 2003)
NDP-085 (2005)
![]() Download the Data and ASCII Documentation files of NDP-085
Download the Data and ASCII Documentation files of NDP-085 ![]() Download a PDF of NDP-085
Download a PDF of NDP-085

Contributed by
E. Peltola,1 R. Wanninkhof,1 R. Feely,2 D. Hansell,3 R. Castle,1 D. Greeley,2 J.-Z. Zhang,1 F. Millero,3 N. Gruber,4 J. Bullister,2 and T. Graham3
Prepared by
Alexander Kozyr
Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, Oak Ridge, Tennessee, U.S.A.
1Atlantic Oceanographic and Meteorological Laboratory, NOAA, Miami, FL
2Pacific Marine Environmental Laboratory, NOAA, Seattle, WA
3Rosentiel School of Marine and Atmospheric Research, Miami University, Miami, FL
4University of California Los Angeles, Los Angeles, FL
Contents
- Abbreviations and Acronyms
- Abstract
- Background Information
- Description of the Expedition
- Description of Variables and Methods
- How to Obtain the Data and Documentation
- References
Abbreviations and Acronyms
| ACRONYM | Definition |
|---|---|
| AOML | Atlantic Oceanographic and Meteorological Laboratory |
| BNL | Brookhaven National Laboratory |
| CCSP | Carbon Cycle Science Program |
| CDIAC | Carbon Dioxide Information Analysis Center |
| CFC | chlorofluorocarbon |
| CLIVAR | Climate Variability (Program) |
| CO2 | carbon dioxide |
| CRM | certified reference material |
| CTD | conductivity, temperature, and depth sensor |
| DOC | dissolved organic carbon |
| DOE | U.S. Department of Energy |
| EXPOCODE | expedition code |
| fCO2 | fugacity of CO2 |
| GCOS | Global Climate Observing System |
| GCRP | Global Change Research Program |
| GOOS | Global Ocean Observing System |
| JGOFS | Joint Global Ocean Flux Study |
| LDEO | Lamont-Doherty Earth Observatory |
| MLR | multilinear regression |
| NDP | numeric data package |
| NDIR | nondispersive infrared analyzer |
| NOAA | National Oceanic and Atmospheric Administration |
| NSF | National Science Foundation |
| ORNL | Oak Ridge National Laboratory |
| pCO2 | partial pressure of CO2 |
| PMEL | Pacific Marine Environmental Laboratory |
| QA | quality assurance |
| QC | quality control |
| RSMAS | Rosentiel School of Marine and Atmospheric Science |
| R/V | research vessel |
| SIO | Scripps Institution of Oceanography |
| SOMMA | single-operator multi-parameter metabolic analyzer |
| SST | sea surface temperature |
| TALK | total alkalinity |
| TCO2 | total inorganic carbon |
| UCI | University of California, Irvine |
| UW | University of Washington |
| WOCE | World Ocean Circulation Experiment |
| WHP | WOCE Hydrographic Program |
Abstract
Peltola, E., R. Wanninkhof, R. Feely, D. Hansell, R. Castle, D. Greeley, J.-Z. Zhang, F. Millero, N. Gruber, J. Bullister, and T. Graham. 2005. Inorganic and Organic Carbon, Nutrient, and Oxygen Data from the R/V Ronald H. Brown Repeat Hydrography Cruise in the Atlantic Ocean: CLIVAR CO2 Section A16N_2003a (4 June - 11 August, 2003), ed. A. Kozyr. ORNL/CDIAC-149, NDP-085. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, Tennessee, 36 pp. doi: 10.3334/CDIAC/otg.ndp085.
This report presents methods and analytical and quality control procedures for nutrient, oxygen, and inorganic carbon system parameters performed during the A16N_2003a cruise, which took place from June 4 to August 11, 2003 aboard NOAA Ship R/V Ronald H. Brown under auspices of the National Oceanic and Atmospheric Administration (NOAA). The first hydrographic leg (June 19 - July 10) was from Reykjavik, Iceland, to Funchal, Madeira, Portugal along the 20°W meridian, and the second leg (July 15 - August 11) continued operations from Funchal, Portugal to Natal, Brazil, on a track southward and ending at 6°S, 25°W. The research was the first in a decadal series of repeat hydrography sections jointly funded by NOAA and the National Science Foundation (NSF) as part of the CLIVAR/CO2/hydrography/tracer program. Samples were taken from up to 34 depths at 150 stations.
The data presented in this report includes the analyses of water samples for total inorganic carbon (TCO2), fugacity of CO2 (fCO2), total alkalinity (TALK), pH, nitrate (NO3), nitrite (NO2), phosphate (PO4), silicate (SiO4), and dissolved oxygen (O2).
The R/V Ronald H. Brown A16N_2003a data set is available free of charge as a numeric data package (NDP) from the Carbon Dioxide Information Analysis Center (CDIAC). The NDP consists of the oceanographic data files and this printed documentation, which describes the procedures and methods used to obtain the data.
Keywords: carbon dioxide, total CO2, total alkalinity, pH, fugacity of CO2, carbon cycle, coulometry, potentiometry, hydrographic measurements, CLIVAR, Atlantic Ocean
1. Background Information
The cruise of research vessel (R/V) Ronald H. Brown along section A16N from Reykjavik, Iceland, to Natal, Brazil, was the first in a series of repeat hydrography cruises to measure decadal changes in circulation, heat and fresh water budgets, and carbon inventory in the ocean. The cruises repeat a subset of the World Ocean Circulation Experiment Hydrographic Program (WHP) and Joint Global Ocean Flux Study (JGOFS) lines occupied in each major ocean basin in the 1990s.
The WOCE/WHP program is driven by the need to monitor the changing patterns of carbon dioxide (CO2) in the ocean and provide the necessary data to support continuing model development that will lead to improved forecasting skills for oceans and global climate. During the 1990s, the WOCE/JGOFS survey provided a full-depth baseline data set against which to measure future changes. By integrating the scientific needs of programs requiring measurement of the full water column, major synergies and cost savings are achieved. These measurements are of importance both for major research programs, such as Climate Variability (CLIVAR) and the U.S. Global Carbon Research Project (GCRP) Carbon Cycle Science Program (CCSP), and for operational activities such as the Global Ocean Observation System (GOOS) and the Global Climate Observing System (GCOS). As outlined in the program documentation one component of a global observing system for the physical climate/CO2 system should include periodic observations of hydrographic variables, CO2 system parameters, and other tracers. The large-scale observation component of CCSP has a need for systematic observations of the invasion of anthropogenic carbon in the ocean that is superimposed on a variable natural background. The five topical areas addressed by the CO2/CLIVAR repeat hydrography program are
- carbon system studies;
- heat and freshwater storage and flux studies;
- deep and shallow water mass and ventilation studies;
- calibration of autonomous sensors; and
- data for model calibration.
The Ronald H. Brown cruise consisted of a transit leg from Charleston, South Carolina, USA to Reykjavik, Iceland, during which limited surface-water observations were taken. The first hydrographic leg was from Reykjavik to Funchal, Madeira, Portugal along the 20°W meridian; the second leg continued operations from Funchal, Portugal to Natal, Brazil, on a track southward, ending at 6°S, 25°W (see Fig. 1.1).
This data report focuses on the measurements of total inorganic carbon (TCO2), fugacity of CO2 (fCO2), total alkalinity (TALK), pH, nitrate (NO3), nitrite (NO2), phosphate (PO4), silicate (SiO4), and dissolved oxygen (O2).
The methodology, instrumentation, and standardization of these parameters improved significantly during the WOCE/JGOFS era. Notable developments include release of manuals detailing the analytical methods and operating protocols (DOE 1994). Certified reference materials (CRMs) are now available for TCO2 and TALK, which are run interspersed with samples to determine calibration offsets. For this cruise the TALK values were adjusted to account for the small difference between the CRMs run at sea and the certified value determined at Scripps Institution of Oceanography (SIO). For TCO2 there were problems with the gas loop calibrations that were attributed to inaccurate temperature sensors. The reference materials were therefore used as primary calibration for both TCO2 and TALK.
Instrumentation also improved in the last decade. Alkalinity measurements can be done with better precision through automation and close checks of the response of electrodes. Burettes are independently calibrated, and the preparation of titrant (hydrochloric acid) undergoes improved quality control and standardization (Millero et al. 1998). Measurement of pH is now done with extreme precision with spectrophotometric methods (Byrne and Breland 1989). The TCO2 measurements are done by coulometry, a precise integrative method. During the A16_2003a cruise we utilized two single-operator multiparameter metabolic analyzers (SOMMAs) (Johnson et al. 1999) for analyses, which facilitated a sample throughput of up to 80 samples per day. The fCO2 measurements were done with an equilibration system described in Wanninkhof and Thoning (1993). For this cruise data reduction and calculation routines were changed. Comparisons of the data with those from a cruise along a similar transect in 1993 show an appreciable bias between results, as is detailed in the section describing the pCO2 analyses. Oxygen measurements were performed by Winkler titrations (Carpenter 1965) with photometric endpoint detection (Friederich, Sherman, and Codispoti 1984). The titrator worked well, but there were issues with errors in bottle volumes and problems with pipettes used to generate standard curves. Extensive post-cruise troubleshooting and bottle volume redetermination were necessary to reduce the data.
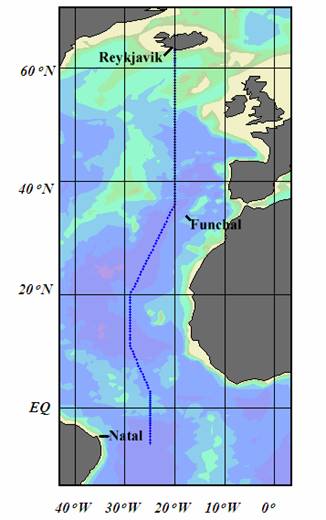
Fig. 1.1. Cruise track for the Atlantic Ocean A16N_2003a cruise in June - August 2003.
The data underwent careful quality assurance and quality control (QA/QC) both during and after the cruise. The precision of the measurements was determined from duplicate sampling and comparison of data from deep water, where little variability is expected. Outliers in the data were flagged based on several methods utilizing prior knowledge of the trends and known relationships between parameters. Depth profiles for each parameter were scrutinized for outliers. When deviations were observed, other parameters were assessed to determine whether they showed deviations as well. Inorganic carbon system parameters were linked through physical and chemical properties; if two of the four parameters are measured, the other two can be calculated, provided that the silicate composition, the phosphate composition, the temperature, and the salinity of the sample are known. These so-called overdeterminations or internal consistency calculations were used to assess the difference between calculated and measured values. When the difference between the measured TALK and the TALK calculated from TCO2 and pH or fCO2 exceeded 10 µmol/kg, the three parameters were scrutinized and compared with other parameters to assess whether the datum should be labeled as questionable. Other techniques, described in detail below, include regional multilinear regressions (MLR) between the inorganic carbon parameters and physical and chemical parameters known to correlate with them [for instance, TCO2 = f(T, S, AOU, Si, PO4)]. Again the differences between measured and calculated parameters were inspected. Finally, the parameters were plotted against latitude for narrow depth intervals. Since changes along depth horizons are usually gradual, anomalies can be easily spotted and flagged.
This report describes procedures and methods for hydrographic measurement and the analytical procedures, calculations, and assessment of precision for nutrient, oxygen, TCO2, TALK, fCO2, and pH measurements. A description of the QA/QC methods based on internal consistency of these parameters and the MLR technique is also provided.
2. Description of the Expedition
2.1 R/V Ronald H. Brown: Technical Details and History
The National Oceanic and Atmospheric Administration (NOAA) ship R/V Ronald H. Brown, a state-of-the-art oceanographic and atmospheric research platform, is the largest vessel in the NOAA fleet. With its highly advanced instruments and sensors, R/V Ronald H. Brown travels worldwide supporting scientific studies to increase our understanding of the worlds oceans and climate. Commissioned on July 19, 1997, in its home port of Charleston, South Carolina, Ronald H. Brown has sailed in the Pacific, Atlantic, and Indian Oceans. The ship was named in honor of former Secretary of Commerce Ronald H. Brown, who was killed in a plane crash on April 3, 1996, while on a trade mission to Bosnia. R/V Ronald H. Brown is operated by NOAA Marine and Aviation Operations and carries a complement of 6 NOAA Corps officers, 20 crew members, and a maximum of 33 scientists. Table 2.1 provides a detailed description of the ship.
| Designer | VT Halter Marine, Inc. |
| Builder | VT Halter Marine, Inc., Moss Point, Mississippi |
| Launched | May 30, 1996 |
| Delivered | April 18, 1997 |
| Commissioned | July, 19, 1997 |
| Hull number | R104 |
| Call letters | WTEC |
| Home port | Charleston, South Carolina |
| Length | 83.5 m (274 ft) |
| Breadth (molded) | 16.0 m (52.5 ft) |
| Draft, maximum | 5.2 m (17.0 ft) |
| Depth to main deck | 8 m (26.5 ft) |
| Displacement | 3,250 tons |
| Maximum speed | 15 kn |
| Cruise speed | 12 kn |
| Range | 11,300 nm at 12 kn speed plus 30 days on station |
| Total crew and scientists | 58 |
| Maximum cruise duration | 60 days |
| Science quarters | Main lab: 1,730 ft2
Electronics/computer lab: 720 ft2 Wet lab: 230 ft2 Hydro lab: 700 ft2 Biochemical lab: 720 ft2 |
| Ship name | Ronald H. Brown |
| EXPOCODE | 33RO200306_01_02 |
| WOCE section | A16N |
| Ports of call | Reykjavik, Iceland, Funchal, Madeira, Portugal and Natal, Brazil |
| Dates | June 4 - August 11, 2003 |
| Funding support | NOAA, NSF |
| Chief scientists | Dr. John L. Bullister, NOAA/PMEL
Dr. Niki Gruber, UCLA |
| Parameter | Institution | Responsible personnel |
|---|---|---|
| CTD, salinity, CTD/O2 | PMEL | G. Johnson |
| Nutrients | PMEL, AOML | C. Mordy, J.-Z. Zhang |
| Oxygen | AOML | J.-Z. Zhang |
| CFCs | PMEL, UW | J. Bullister, M. Warner |
| Tritium, Helium | LDEO | P. Schlosser |
| TCO2, fCO2 | AOML, PMEL | R. Wanninkhof, R. Feely |
| TALK, pH | RSMAS/UM | F. Millero |
| DOC | RSMAS/UM | D. Hansell |
| 14C, 13C | UCI | E. Saltzman |
| PMEL | - | Pacific Marine Environmental Laboratory |
| AOML | - | Atlantic Oceanographic and Meteorological Laboratory |
| LDEO | - | Lamont-Doherty Earth Observatory |
| RSMAS/UM | - | Rosentiel School of Marine and Atmospheric Science, University of Miami |
| UCI | - | University of California, Irvine> |
| UW | - | University of Washington |
3. Description of Variables and Methods
3.1 Nutrient and Oxygen Measurements
The analytical method for determining dissolved oxygen in seawater during the A16N_2003a cruise was based on automated Winkler titration as described by Culberson et al. (1991), and Williams and Jenkinson (1982) and modified by Friederich, Codispoti, and Sakamoto (1991). Dissolved oxygen samples were withdrawn from 10-L Niskin bottles to 145-mL Pyrex brand iodine flasks (Corning 5400, Corning, New York, USA). The exact volume of each flask at room temperature had been gravimetrically calibrated with its ground glass stopper following standard procedures (DOE 1994; WHP Operations and Methods 1991). Quantities of 1 mL of manganese chloride reagent and 1 mL of alkaline iodide reagent were added to each sample in the iodine flasks, and then the stopper was placed in the bottle neck. The bottles were shaken vigorously for about 1 min to completely fix oxygen with manganese hydroxide [Mn(OH)3]. In this method, dissolved oxygen in the sample reacts with Mn(OH)3 to form Mn(OH)3 precipitate. Particulate Mn(OH)3 dissolves upon the acidification, and the resulting Mn3+ ions oxidize iodide to iodine in acidic solution. The liberated iodine complexes with excess iodide, forming I3, and the latter is titrated with a sodium thiosulfate solution that is standardized by a primary standard potassium iodate. The complex I3 has a maximum absorbance at 352 nm; this change in absorbance at 352 nm is used to detect the end point. A custom-built automated oxygen titrator with MS DOS interfacing software was used to determine dissolved oxygen concentrations in the samples.
A total of 5011 seawater samples were taken from 150 stations and analyzed for dissolved oxygen concentrations. At the beginning of cruise, a test conductivity, temperature, and depth (CTD) cast was made by sampling 20 Niskin bottles from same depth (170 m). Analysis of these samples indicated a precision of 0.3 µmol/L. Throughout the cruise duplicate samples from the same Niskin bottle were collected at each station to estimate the precision of the overall measurements (sampling and analysis). Analyses of 300 replicate samples indicated that the precision of the shipboard automated Winkler titration was 0.29 µmol/L including all outliers, and 0.24 µmol/L excluding the outliers. An analysis of outliers indicated that most outlying values in the duplicate analysis were due to errors in the volumes of oxygen bottles; a minority were due to problems with Niskin bottles or sampling error. The outliers in vertical profiles of oxygen were also used to identify the bottles that might have errors in volumes. A total of 33 sample bottles were recalibrated; 11 proved to have volume errors greater than 0.3 mL. These accounted for about 5% of the sample bottles used during the A16N_2003a cruise. The volumes of the oxygen bottles identified as questionable were recalibrated after the cruise, and dissolved oxygen concentrations were recalculated for those samples using correct volumes.
At the beginning of leg 2 of the cruise (from stations 72 to 79) a problematic automatic pipette was used to deliver the KIO3 standard solution for standardization of thiosulfate solution in batch number 14. An unusually high slope was observed, and this pipette was not used in subsequent analyses. A shipboard and post-cruise comparison indicated that there was an error in volume delivery of this automatic pipette. Consequently, dissolved oxygen concentrations from stations 72 to 79 were corrected for errors in volume delivery of iodate solution by this automatic pipette. The correction factor (1.0153) was estimated on the basis of the post-cruise recalibration of the pipette and was applied to data from station 72 to 79.
Since the Dosimat titrators have demonstrated high precision and accuracy in volume delivery of titrants (0.05 and 0.2%, respectively, at delivery of 10 mL solution), we recommend use of a Dosimat or a similar positive-displacement burette to quantitatively dispense the iodate standard solution during future cruises. This procedure can improve the accuracy of shipboard oxygen analysis.
Nutrient samples were collected from Niskin bottles in acid-washed 25-mL linear polyethylene bottles after at least three complete seawater rinses and analyzed within 2 hours of sample collection. Measurements were made in a temperature-controlled (20 ± 2°C) bioanalytical laboratory aboard the R/V Ronald H. Brown. Concentrations of nitrite (NO2), nitrate (NO3), phosphate (PO43), and silicic acid (H4SiO4) were determined using a modified Alpkem Flow Solution Auto-Analyzer coupled with a modified RFA 301 autosampler. Sample and wash time for the auto sampler were set at 120 and 5 seconds, respectively.
Nitrite content was determined by diazotizing the samples with sulfanilamide and bonding with N-1 naphthyl ethylenediamine dihydrochloride to form an azo dye. The color produced is measured at 540 nm (Zhang, Ortner, and Fischer 1997). Samples for nitrate analysis were passed through a laboratory-crafted cadmium column (Zhang, Fischer, and Ortner 2000) to reduce nitrate to nitrite. Total nitrite, mostly from reduction of nitrate with a small amount of nitrite present in the original samples, was then determined as described above. Nitrate concentrations in seawater samples were calculated by difference.
The amount of phosphate in the samples was determined by reacting the samples with molybdenum (VI) in an acidic medium to form a phosphomolybdate complex. This complex was subsequently reduced with hydrazine at a temperature of 55°C to form phosphomolybdenum blue (Zhang, Fischer, and Ortner 2001). An AAII detector with an 880-nm filter was used to measure the absorbance during the cruise.
Silicic acid in the samples was analyzed by reacting samples with molybdate in a acidic solution to form b-molybdosilicic acid. The b-molybdosilicic acid was then reduced by ascorbic acid to form molybdenum blue (Zhang and Berberian 1997). The absorbance of the molybdenum blue was measured at 660 nm.
The low-nutrient water used for the preparation of working standards, determination of blank, and the wash between samples was filtered seawater obtained from the surface of the Gulf Stream. Stock standard solutions were prepared by dissolving high-purity standard materials (KNO3, NaNO2 , KH2PO4 and Na2SiF6 ) in deionized water. Working standards were freshly made at each station by diluting the stock solutions in low-nutrient seawater. Standardizations were performed prior to each sample run with working standard solutions. Two or three replicate samples were collected from a Niskin bottle that tripped at the deepest depth at each cast. The relative standard deviation from the results of these replicate samples was used to estimate the overall precision for the sampling and analytical procedures. The precision of analyses was 0.08 µmol/kg for nitrate, 0.01 µmol/kg for phosphate, and 0.1 µmol/kg for silicic acid, respectively.
3.2 Total Inorganic Carbon Measurements
Samples were drawn from the Niskin bottles into cleaned, precombusted 540-mL Pyrex bottles using Tygon tubing according to procedures outlined in DOEs handbook of methods for CO2 analysis (DOE 1994). Bottles were rinsed once and filled from the bottom, overflowing half a volume. Care was taken not to entrain any bubbles. The tube was pinched off and withdrawn, creating a 5-mL headspace; and 0.2 mL of saturated HgCl2 solution was added as a preservative. The sample bottles were sealed with glass stoppers lightly covered with Apiezon-L grease and were stored at room temperature for a maximum of 12 hours prior to analysis.
The TCO2 analytical equipment was set up in a seagoing laboratory van. The analysis was done by coulometry with two analytical systems (AOML-1 and AOML-2) used simultaneously on the cruise. Each system consisted of a coulometer (UIC, Inc.) coupled with a SOMMA inlet system developed by Ken Johnson, formerly of Brookhaven National Laboratory (Johnson 1992; Johnson, King, and Sieburth 1985; Johnson et al. 1987, 1993). In the coulometric analysis of TCO2, all inorganic carbon is converted to CO2 (gas) by the addition of excess hydrogen ions (acid) to the seawater sample, and the evolved CO2 gas is swept into the titration cell of the coulometer with compressed nitrogen, where it reacts quantitatively with a proprietary reagent based on ethanolamine to generate hydrogen ions. These are subsequently titrated with coulometrically generated OH. Carbon dioxide is quantified by integrating the total charge required to achieve this.
The coulometers were calibrated by injecting aliquots of pure CO2 (99.995%) by means of an eight-port valve outfitted with two sample loops that had been calibrated by Kelly Brown, CCN Consulting (Wilke, Wallace, and Johnson 1993). Due to large temperature variations of the gas loops, the calibration factors obtained from gas loop measurements were of poor quality. Instead of using an average of the small and large loop values as recommended in DOE (1994), we used a constant value for each analyzer throughout the entire cruise. The constant calibration value used for AOML-1 was 1.00532 and for AOML-2 1.00650. The CO2 gas volumes bracketed the amount of CO2 extracted from the water samples for the two AOML systems. All TCO2 values were corrected for dilution by 0.2 mL of HgCl2 used for sample preservation. The correction factor used for dilution was 1.00037. A correction was also applied for the offset from the Certified Reference Material (CRM) Batch 59, supplied by Dr. A. Dickson (SIO). This correction was applied for each cell using the CRM value obtained for the cell at the beginning. To check the stability of the coulometer and coulometer solutions, the CRMs were measured at the beginning, middle, and end of each coulometer cell solution. The coulometer cell solution was replaced after 25 mg of carbon was titrated, typically after 912 hours of continuous use. Sample titration times were 916 minutes.
Replicate seawater samples were taken from Niskin sample bottles at the surface, at 1000 m, and at the sea bottom and were run at different times during the cell. The first replicate of the surface water was used at the start of the cell with fresh coulometer solution; the second surface replicate and the first one of the 1000 replicates were run in the middle of the cell after about 12 mg of carbon were titrated. The second one of the 1000 m replicates and the first one of the bottom replicates were run at the end of the cell after about 25 mg of carbon were titrated, while the second one of the bottom replicate samples was run using a new coulometer cell solution. No systematic difference between the replicates was observed. The trends do not suggest any systematic dependency of results with amount of carbon titrated for a particular cell. The results of the duplicate samples are shown in Fig. 3.1 and Table 3.1.
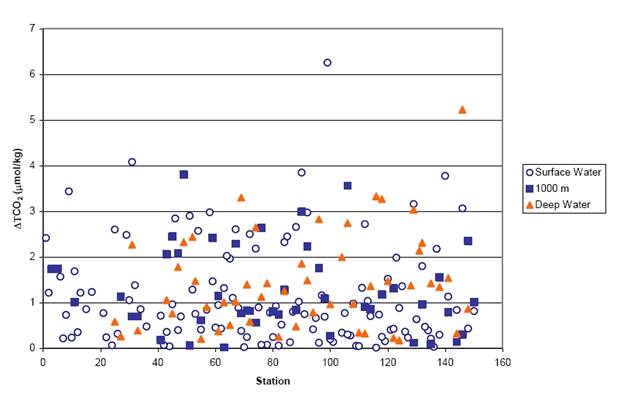
Fig. 3.1. Results of the duplicate TCO2 samples collected during the R/V Ronald H. Brown cruise along the Atlantic Ocean section A16N_2003a.
| Measurement method | Av. | Std. dev. | No. |
|---|---|---|---|
| Duplicate samples measured back-to-back | 0.8 | 0.80 | 94 |
| One duplicate measured in the beginning; the other in the middle of the cell | 1.3 | 0.94 | 39 |
| One duplicate measured in the middle; the other at the end of the cell> | 1.2 | 0.57 | 13 |
| One duplicate measured in the beginning; the other, at the end of the cell | 1.3 | 1.27 | 8 |
| Duplicates run on the same instrument but on different cells | 1.4 | 0.86 | 56 |
| Duplicates run on different instruments | 0.7 | 0.42 | 3 |
| Duplicates measured in the beginning of the cell, but not back-to-back | 0 | ||
| Duplicates measured in the middle of the cell, but not back-to-back | 1.3 | 1.01 | 6 |
| Duplicates were measured in the end of the cell, but not back-to-back | 1.0 | 0.30 | 3 |
| Total no. of measurements | 286 | ||
| Measurements deleteda | 64 | ||
| Measurements used | 222 | ||
aMeasurements that were two standard deviation removed from the mean were omitted from the statistics.
The concentration of TCO2, designated as [CO2], in the samples was determined according to
![]() (3.1)
(3.1)
where Cal. factor is the calibration factor fixed for this cruise because of the malfunctioning of the gas loops, Counts is the instrument reading at the end of the analysis, Blank is the counts per minute determined from blank runs performed at least once for each cell of the solution, Run time is the length of coulometric titration (in minutes), and K is the conversion factor from counts to mol, which is dependent on the slope and intercept relation between instrument response and charge. For a unit with an Ecal slope of 1 and an intercept of 0, the conversion factor is 2.0728 X 10-4.
The blank values for AOML-1 were in the range of 12.0 to 33.3 counts/min, with an average value of 19.6 counts/min and a standard deviation of 6.8 counts/min. For AOML-2 they were in the range of 12.0 to 30.0 counts/min, with an average value of 21.7 counts/min and a standard deviation of 6.1 counts/min.
The pipette volume was determined by taking aliquots of distilled water at a known temperature from the volumes prior to the cruise. The weights with the appropriate densities were used to determine the volume of the pipettes (AOML-1: 28.726 cm3 @ 19.96C, AOML-2: 22.623 cm3 @ 22.63C).
Calculations of pipette volumes, density, and final CO2 concentrations were performed according to procedures outlined in the DOE CO2 handbook (DOE 1994).
3.3 Fugacity of CO2 Measurements
The fugacity of CO2 (fCO2) was measured on the A16N-2003a cruise at a constant temperature of 20°C by equilibrating a 500-mL water aliquot in a volumetric flask with a closed headspace. The headspace is circulated through a nondispersive infrared detector that measures both CO2 and water vapor levels. The analytical instrumentation is detailed in Wanninkhof and Thoning (1993) and is the same as the setup used in the N.Atl-93 cruise that occupied the same cruise line in 1993 (Castle et al. 1998).
The system is patterned after that of Chipman, Marra, and Takahashi (1993) with modifications as presented in Wanninkhof and Thoning (1993). In short, a 500-mL water sample is equilibrated at ambient pressure with an 80-mL headspace in a thermostatted volumetric flask. The headspace is circulated through a nondispersive infrared analyzer (NDIR), LICOR model 6262. Upon equilibration the circulation flow is stopped, and 30 readings of water vapor content and CO2 content in the cell are taken over a 30-second interval and averaged. The system is a dual-channel system where one equilibration occurs while circulating through the NDIR and a second flask is equilibrated offline. Once the first sample is analyzed, the second flask is switched in line with the NDIR, and the gas in the NDIR is equilibrated with the second flask content. The second equilibration phase through the NDIR takes less time, as a large part of the headspace has already been equilibrated offline. The two-channel configuration decreases the total analysis time to about 20 min for two samples.
The system is calibrated after every eight samples with six gaseous standards traceable to the manometrically determined values of C. D. Keeling of SIO. The mole fractions of the standards used during the A16N_2003a cruise were as follows:
| Tank number | Mole fraction (ppm) |
|---|---|
| CA05989 | 378.7 |
| CA05980 | 792.5 |
| CA05984 | 1036.9 |
| CA05940 | 1533.7 |
| CA05988 | 593.6 |
| CA05998 | 205.1 |
The standards were also used as the headspace gas for the equilibration. Since the mole fractions of the gases in the headspace prior to equilibration are known, the small perturbation of fCO2 in the water during the equilibration process can be accounted for quantitatively. The headspace gas is selected such that it is close the anticipated water value, thereby minimizing the correction.
The calculation of fCO2 involves several steps, including the conversion of the NDIR output to an equivalent dried mole fraction of CO2, the correction for the perturbation of fCO2 in water by equilibration, and the small adjustment from the measurement temperature to 20°C. For the reduction of the A16N_2003a fCO2 we made an important adjustment in procedures. On previous cruises, the calibration of the samples that were run at 100% water vapor pressure (@ 20°C) to the dry standards was done through an empirical algorithm created by running standards both wet and dry. For this cruise we relied on the internal correction from wet to dry mole fraction of CO2 provided by the LI-COR 6262. This change is based on testing by our group and other investigators that showed that the correction provided by the instrument is of high quality and subject to less uncertainty than our empirical corrections. Since this is a fundamental change in our procedures, we describe the old and new routine in detail below, including comparison of the results.
The correction from detector output to (dry) mole fraction of CO2, xCO2 in the headspace was previously done by measuring the voltage output of the CO2 and H2O channel. An empirical algorithm between dry standards and standards saturated with water vapor at 20°C was created in the form
MVCO2(dry) = MVCO2(wet) + A + B × MVCO2(wet) + C × [MVCO2(wet)]2 , (3.2)
where MV is the millivolt output of the CO2 channel and MVCO2 (wet) is the millivolt value measured for the equilibrated headspace of the sample. From this algorithm the (water-saturated) headspace gas is corrected to the dry state such that the samples can be directly related to the standard. The next step is to convert the MVCO2(dry) of the sample to an xCO2 by creating a curve of MVCO2(dry) versus xCO2 using the standards preceding and following the samples. For each sample the three standards closest to the samples are selected and a second-order polynomial is created of MVCO2 versus xCO2 by averaging the appropriate standards preceding and following the sample. The second-order polynomial is then used to calculate the xCO2 of the sample.
Following this step the fCO2 in the headspace is calculated according to:
fCO2 = xCO2 (1 − pH2O) × 0.9966 , (3.3)
where pH2O is the water vapor pressure @ 20°C (= 0.0226 atm) and 0.9966 is the conversion factor from pCO2 to fCO2 @ 20°C.
The next step is the correction for change in fCO2 in the water sample due to exchange of CO2 with the headspace during equilibration. This step is accomplished by using the mass balance criterion that the total amount of carbon in the headspace and water is conserved and by using the fact that the TALK remains unchanged during equilibration. The TCO2 of the sample (determined independently) and the headspace gas concentration prior to equilibration along with the volume of water and headspace are used to calculate the total amount of carbon in the system. From the change in headspace CO2 before versus after equilibration, the change the TCO2 in the water can then be determined. From this change and the TALK (calculated from TCO2 and fCO2after equilibration), the fCO2 in the water before equilibration can then be determined.
The final step is to correct the fCO2 from analysis temperature to 20°C. The water samples were always equilibrated within 0.1°C of 20°C such that this correction is less than 0.4% of the value. The correction for perturbation of fCO2 in the water during equilibration and the temperature correction to 20°C are performed using the carbonate dissociation constants and the temperature dependence of the constants and the calculation routines described in Peng et al. (1987).
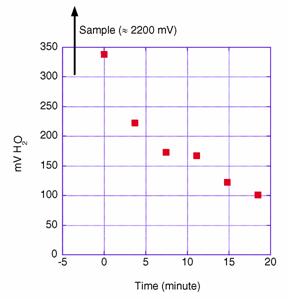
For A16N_2003a the correction from the moist gas of the sample to an equivalent dry concentration was performed utilizing the internal correction routine built into the Li-6262 analyzer. This internal algorithm has been extensively checked by others and our tests also showed that the correction was robust. The important advantage of this internal correction is that in our previous data reductions we assumed that the algorithm between wet and dry created in laboratory tests before the cruise or after the cruise does not change appreciably over time. This has proven to not always be the case. Secondly, the water vapor level measured during the standard runs can be appreciable despite an absence of water vapor in the compressed gas standards, since it takes a long time for the water vapor introduced by the equilibration of the samples to be flushed from the system. Therefore, we see a decreasing trend of water vapor level when the six standards are run consecutively (Fig. 3.2).
The modified data reduction routine uses the xCO2(dry) calculated by the detector for both standards and samples. A second-order polynomial fit is created between the actual mole fraction of CO2 in the standard and the instrument value. This standardization accounts for instrument drifts over time. The detector was zeroed and spanned for CO2 every day, while the water vapor channel was spanned just before the first leg and before the second leg. Standardizing the water vapor channel is difficult because of the stickiness of the water vapor, which leads to lags and very slow response times. A polynomial is created for the three standards closest to the sample by averaging the pertinent standards before and after taking the sample. The other steps of correcting for small temperature deviations of the water bath from 20°C and correction to fCO2 prior to equilibration are identical to the procedures outlined above.
The new correction routine results in small differences in values for calculated fCO2 compared to the previous data reduction routine. Table 3.2 shows a comparison in values for station 45 using the different methods. The values using the new reduction routine are systematically about 2 &mocro;atm lower than those obtained through the old reduction method. The table also gives the results of two different water vapor correction algorithms. One empirical correction was established before the cruise, and one was determined from running wet versus dry standards after the cruise. The results show differences in the range from 7 to 17 µatm.
 /p>
/p>
Fig. 3.2. Change in water vapor concentration (in millivolts) when a set of six (dry) standards are run, showing that some residual water vapor remains in the lines after water samples are equilibrated. (Water samples show an H2O response of about 2200 mV.)
| Keyfield | Lat. (N) | Pressure | fCO2(20) Finala | Cruiseb | New H20c |
|---|---|---|---|---|---|
| 45101 | 43 | 5239.7 | 762.9 | 765.80 | 745.8 |
| 45102 | 43 | 4994.3 | 765.7 | 768.80 | 748.5 |
| 45103 | 43 | 4499.7 | 769.5 | 771.45 | 751.7 |
| 45104 | 43 | 3983.9 | 768.5 | 770.30 | 751.8 |
| 45106 | 43 | 3001.5 | 758.4 | 760.50 | 742.1 |
| 45108 | 43 | 2000.5 | 755.2 | 756.60 | 738.6 |
| 45109 | 43 | 1800.0 | 761.4 | 762.90 | 745.3 |
| 45111 | 43 | 1401.5 | 746 | 747.80 | 729.8 |
| 45112 | 43 | 1200.0 | 728.4 | 730.10 | 712.9 |
| 45114 | 43 | 1001.0 | 724.1 | 725.70 | 708.1 |
| 45115 | 43 | 900.3 | 728.7 | 730.40 | 713.2 |
| 45116 | 43 | 800.7 | 712.4 | 714.00 | 696.6 |
| 45117 | 43 | 699.6 | 712.3 | 713.80 | 696.9 |
| 45118 | 43 | 601.3 | 687.2 | 689.00 | 672.7 |
| 45119 | 43 | 501.0 | 635.2 | 637.20 | 621.3 |
| 45121 | 43 | 401.1 | 576.8 | 578.60 | 563.8 |
| 45123 | 43 | 299.7 | 556.3 | 557.90 | 543.4 |
| 45125 | 43 | 201.0 | 510.7 | 512.10 | 499.1 |
| 45127 | 43 | 151.0 | 507.8 | 509.00 | 495.7 |
| 45129 | 43 | 99.7 | 494.1 | 495.30 | 482.3 |
| 45130 | 43 | 79.6 | 486.6 | 487.80 | 474.8 |
| 45131 | 43 | 60.0 | 482.2 | 483.40 | 471.7 |
| 45132 | 43 | 39.5 | 450.7 | 451.80 | 440.2 |
| 45133 | 43 | 19.9 | 381.9 | 384.20 | 374.2 |
| 45135 | 43 | 3.4 | 374.7 | 375.30 | 365.6 |
aFinal data reduction using the detector xCO2 (dry) output.
bData reduction on cruise using an empirical water vapor correction.
cData reduction in Jan. 2004 using an empirical water vapor correction that was determined after the cruise.
During the cruise a total of 1515 Niskin samples were analyzed for fCO2, compared with 2500 TCO2 samples. This was because only one full-time and a part-time operator were available for the work, while two full-time analysts were involved in TCO2 analysis. A summary of the analysis statistics is given in Table 3.3.
| Total number of stations | 150 |
| Total number of stations sampled for fCO2 (full depth) | 67 |
| Total number of Niskin bottles tripped | 4823 |
| Total number of Niskin bottles sampled for fCO2 | 1522 |
| Number of duplicates | 140 |
| Number of bad values | 6 |
| Number of questionable values | 48 |
The precision of the results is based on comparison of duplicate values and is estimated to be 2 µatm, or 0.3%. There is no apparent trend in imprecision with depth or absolute concentration when comparing absolute difference. The relative (percentage) difference is slightly higher for lower fCO2 values found near the surface.
The A16N_2003a cruise overlapped or intersected with two previous cruises that were sampled by the AOML group. The NAtl-93 cruise (Castle et al. 1998) followed the same track and was occupied during the summer of 1993 but the section was run from south to north. A 24-bottle rosette was used such that fewer depth samples were obtained and the spacing of the stations was nominal at 1, compared to 0.5 spacing on the 2003 occupation.
The 24N-98 cruise was run in February 1998 and intersected the A16N-2003a section near 24°N, 26.5°W. In the comparison we make the assumption that changes in deep water are negligible over the time period. The crossover with the 24°N cruise is shown in Fig. 3.3. The fCO2 shows a consistent offset, with the 2003 data being about 18 µatm higher than the 1998 data. For the comparison with the 1993 data we looked at the deep-water measurements for stations spaced about 5° apart (Fig. 3.4). Again, a systematic bias is observed, with the 2003 data being higher. The magnitude of the bias, however, is about 10 µatm. The cause of these disconcerting offsets is attributed to the water vapor correction. However, the exact reason or possible corrections is not readily apparent.
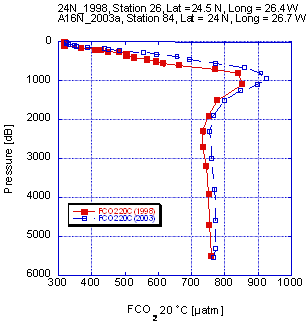
Fig. 3.3. Comparison of fCO2 (20) profiles for a crossover location between a cruise in 1998 and the A16N_203a cruise.
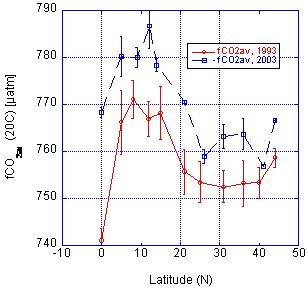
Fig. 3.4. Comparison of deep-water fCO2 values for a cruise in 1993 and the A16N_2003a cruise at the depth range between 4000 and 5000 m.
The surface-water fCO2 levels were measured with a different system in underway mode near sea surface temperature and offered an independent assessment of the agreement of fCO2 values. However, the temperature correction has some uncertainties which complicate the comparison. For the comparison, the fCO2(20) values were corrected to a sea-surface temperature (SST) as determined by the thermosalinograph using the empirical correction of ∂fCO2/∂T = 0.0423°C-1 and by using the temperature dependence of the dissociation constant and using the thermodynamic equations of Mehrbach as refit by Dickson and Millero. The results, shown in Fig. 3.5 show the following average differences:
- −3.30 ± 4.9 µatm (n = 76) ) for fCO2(UW) − fCO2(disc)Mehr
- −6.66 ± 4.1 µatm (n = 76) for fCO2(UW) − fCO2(disc)4.23%.
In the case of fCO2(UW) − fCO2(disc)Mehr, the fCO2(20) are normalized to sea-surface temperature using the Mehrbach constants as refit by Dickson and Millero. For fCO2(UW) − fCO2(disc)4.23%, the fCO2(20) are normalized to SST using the empirical relationship of 0.0423°C-1. Again, our temperature-corrected discrete data are on average higher than the underway measurements. The differences fCO2(UW) − fCO2(disc)Mehr and fCO2(UW) − fCO2(disc)4.23% are plotted against temperature in Fig. 3.6. There is a slight trend with temperature for the adjustments using the Mehrbach constants. Also, near 20°C, when the adjustment is small, the comparison shows that the discrete data is systematically higher. For the range from 18 to 22°C the difference is
- −5.1 ± 4.9 µatm (n = 76) for fCO2(UW) − fCO2(disc)Mehr
- −6.7 ± 4.1 µatm (n = 76) for fCO2(UW) − fCO2(disc)4.23%
very similar to the average difference over the entire temperature range, suggesting that the systematic offset is not attributable to the temperature correction alone.
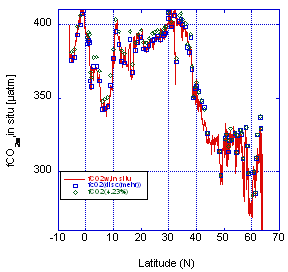
Fig. 3.5. Comparison of underway fCO2 measurements (line) with the discrete samples normalized to the same temperature as the underway measurements using an empirical relationship of 4.23% °C-1 (diamonds) and the constants of Mehrbach (open squares).
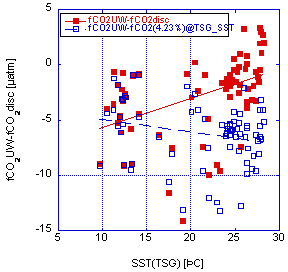
Fig. 3.6. Difference in underway fCO2 measurements and with the discrete samples normalized to the same temperature as the underway measurements using an empirical relationship of 4.23% °C-1 (open squares) and the constants of Mehrbach (solid squares).
3.4 Total Alkalinity Measurements
Seawater samples for TALK were drawn from the Niskin bottles with a 40-cm length of silicon tubing. One end of the tubing was fit over the petcock of the Niskin bottle, and the other end was inserted into the bottom of a 500-mL Corning glass-stoppered sample bottle. The sample bottle was rinsed three times with approximately 300 mL of seawater. The sample bottle was slowly filled from the bottom. Once filled, the sample bottles were kept in a constant water bath at 25°C for a half-hour before analysis.
The titration system used to determine TALK consisted of a Metrohm 665 Dosimat titrator and an Orion 720A pH meter controlled by a personal computer (Millero et al. 1993). The acid titrant, in a water-jacketed burette, and the seawater sample, in a water-jacketed cell, were kept at 25 ± 0.1°C with a Neslab constant-temperature bath. The Plexiglas water-jacketed cells were similar to those used by Bradshaw and Brewer (1988), except that a larger volume (200 mL) was used to increase the precision. The cells had fill and drain valves with zero dead-volume to increase the reproducibility of the cell volume.
The HCl solutions used throughout the cruise were made, standardized, and stored in 500-mL glass bottles in the laboratory for use at sea. The 0.23202 M HCl solutions were made from 1 M Mallinckrodt standard solutions in 0.45 M NaCl to yield an ionic strength equivalent to that of average seawater (0.7 M). The acid was standardized using a coulometric technique by the University of Miami and by Dr. Dickson of SIO. The two standardization techniques agreed to 0.0001 N.
The volume of HCl delivered to the cell is traditionally assumed to have a small uncertainty (Dickson 1981) and is equated with the digital output of the titrator. Calibrations of the Dosimat burettes with Milli Q water at 25°C indicated that the systems deliver 3.000 mL (the value for a titration of seawater) to a precision of 0.0004 mL. This uncertainty resulted in an error of 0.4 µmol/kg in TALK.
The titrators were calibrated in the laboratory before the cruise. CRM Batch 59, prepared by Dr. Dickson, was used at sea to monitor the performance of the titrators. All TALK data have been corrected based on CRM values for each cell and each leg (Millero et al. 2000); see Table 3.4.
| Leg | TALK (µmol/kg) | TCO2 (µmol/kg) | pH (total scale) @ 25°C | Total no. of runs |
|---|---|---|---|---|
| Leg 1 | ||||
| System 1 | 2222.2 ± 3.6 | 2015.0 ± 3.7 | 7.891 ± 0.007 | 15 |
| System 2 | 2224.2 ± 3.2 | 2017.7 ± 3.4 | 7.893 ± 0.007 | 17 |
| Leg 2 | ||||
| System 1 | 2222.5 ± 4.5 | 2012.1 ± 2.4 | 7.895 ± 0.009 | 16 |
| System 2 | 2222.9 ± 3.8 | 2016.1 ± 4.1 | 7.890 ± 0.009 | 15 |
| Manual system | 2217.2 ± 2.1 | 2013.4 ± 0.5 | 7.888 ± 0.006 | 3 |
| Both Legs | ||||
| System 1 | 2222.4 ± 3.8 | 2013.6 ± 3.4 | 7.891 ± 0.011 | 33 |
| System 2 | 2223.6 ± 3.5 | 2017.0 ± 3.8 | 7.891 ± 0.008 | 30 |
| Manual system | 2217.2 ± 2.1 | 2013.4 ± 0.5 | 7.888 ± 0.006 | 3 |
| All systems | 2222.7 ± 3.6 | 2015.2 ± 3.5 | 7.891 ± 0.009 | 66 |
| Certified Values | ||||
| CRM Batch 59 | 2220.98 | 2007.1 | 7.895a | |
| 7.9674±0.0005b | 19 | |||
| TRIS | 8.0855±0.0003a | 19 | ||
| Correction Factor, Leg 1 | ||||
| System 1 | 0.9994 | 0.9961 | 0.004 | |
| System 2 | 0.9980 | 0.9947 | 0.002 | |
| Correction Factor, Leg 2 | ||||
| System 1 | 0.9988 | 0.9975 | 0.000 | |
| System 2 | 0.9991 | 0.9958 | 0.005 | |
| Manual system | 1.0017 | 0.9969 | 0.007 | |
3.5 pH Measurements
Seawater samples were drawn from the Niskin bottles with a 20-cm length of silicon tubing. One end of the tubing was fit over the petcock of the Niskin bottle, and the other end was attached over the opening of a 10-cm glass spectrophotometric cell. The spectrophotometric cell was rinsed three to four times with a total volume of approximately 200 mL of seawater; the Teflon endcaps were also rinsed and then used to seal a sample of seawater in the glass cell. While drawing the sample, care was taken to make sure that no air bubbles were trapped within the cell. The sample cells were kept in a water bath at 20°C for a half an hour before analysis.
Seawater pH was measured using the spectrophotometric procedure (Byrne 1987) and the indicator calibration of Clayton and Byrne (1993). The indicator was an 8.0-mM solution of m-cresol purple sodium salt (C21H17O5Na) in MilliQ water.
The absorbance measurements were made using a Varian Cary 2200 spectrophotometer. The temperature was controlled to a constant temperature of 25°C with an Endocal RTE 8DD refrigerated circulating temperature bath that regulates the temperature to 0.01°C. The temperature was measured using a Guildline 9540 digital platinum resistance thermometer.
3.6 Total Organic Carbon and Total Nitrogen Measurements
Water samples were collected from the rosette. Samples collected from the surface to 250 metes were filtered using precombusted (450°C) GF/F inline filters as they were being collected from the Niskin bottle. At depths greater than 250 meters the samples were collected without filtration. After collection, samples were frozen upright in 60 mL acid-cleaned HDPE bottles, and remained cold until analysis. Prior to analysis, samples were returned to room temperature then acidified to pH < 2 with concentrated hydrochloric acid. Analysis was performed using a Shimadzu TOC-VCSH Total Organic Carbon Analyzer with the TNM-1 Total Nitrogen detector. Instrument conditions were as follows:
| Combustion Temperature | - | 680°C |
| Carrier Gas | - | UHP Oxygen |
| Carrier Flow Rate | - | 150 mL/min |
| Ozone Generation Gas | - | Zero Air from Whatman TOC Gas Generator |
| Ozone Flow Rate | - | 500 mL/min |
| Sample Sparge time | - | 2.0 minutes |
| Minimum number of injections | - | 3 |
| Maximum number of injections | - | 5 |
| Number of Washes | - | 2 |
| Standard Deviation Maximum | - | 0.1000 |
| CV Maximum | - | 2.00% |
| Injection Volume | - | 100 µL |
The TOC system was calibrated using potassium hydrogen phthalate in Milli-Q water and the TN system was calibrated using potassium nitrate in Milli-Q water. System performance was verified daily using Consensus Reference Water. This reference water is deep Sargasso Seawater that has been acidified and sealed in 10 mL ampoules, the concentration of which has been determined by the consensus of up to six expert and independent laboratories. After verifying proper operation of the TOC/TN instrument, samples were set up on an auto sampler for analysis. The run started with a QW (Q Water) blank and a reference seawater analysis. Then six samples would be analyzed followed by another QW blank and reference seawater. This sequence would be repeated until all samples for that run were analyzed. The run ended with a QW blank, reference water and a QW blank that had not been acidified. This was done to verify that the hydrochloric acid used to acidify the samples was not contaminated. QW blanks and reference water samples were used to evaluate system performance during the analytical run. If a problem was detected with the blanks or reference waters, the samples were reanalyzed.
4. How to Obtain the Data and Documentation
This database (NDP-085) is available free of charge from CDIAC. The data are available from CDIAC's anonymous file transfer protocol area. The complete documentation and data can be obtained from the CDIAC oceanographic Web site.
For additional information, contact CDIAC.
5. References
- Bradshaw, A. L., and P. G. Brewer. 1988. High-precision measurements of alkalinity and total carbon dioxide in seawater by potentiometric titration-1: Presence of unknown protolyte(s)? Maine Chemistry 23:69-86.
- Byrne, R. H. 1987. Standardization of standard buffers by visible spectrometry. Analytical Chemistry 59:1479-81.
- Byrne, R. H., and J. A. Breland. 1989. High-precision multiwavelenth pH determinations in seawater using cresol red. Deep-Sea Research 36:803-10.
- Carpenter, J. H. 1965. The Chesapeake Bay Institute technique for the Winkler dissolved oxygen method. Limnology and Oceanography 10:141-43.
- Castle, R., R. Wanninkhof, S. C. Doney, J. Bullister, L. Johns, R. A. Feely, B. E. Huss, F. J. Millero, and K. Lee. 1998. Chemical and hydrographic profiles and underway measurements from the North Atlantic during July and August of 1993. NOAA Data Report ERL AOML-32, National Oceanic and Atmospheric Administration, Atlantic Oceanographic and Meteorological Laboratory, Springfield, N.J.
- Chipman, D. W., J. Marra, and T. Takahashi. 1993. Primary production at 47N and 20W in the North Atlantic Ocean: A comparison between the 14C incubation method and mixed layer carbon budget observations. Deep-Sea Research II 40:151-69.
- Clayton T., and R. H. Byrne. 1993. Calibration of m-cresol purple on the total hydrogen ion concentration scale and its application to CO2-system characteristics in seawater. Deep-Sea Research 40:2115-29.
- Culberson, C. H., G. Knapp, M. Stalcup, R. T. Williams, and F. Zemlyak. 1991. A Comparison of Methods for the Determination of Dissolved Oxygen in Seawater. WHP Office Report, WHPO 91-2. WOCE Hydrographic Program Office, Woods Hole, Mass.
- Dickson, A. G. 1981. An exact definition of total alkalinity and a procedure for the estimation of alkalinity and total CO2 from titration data. Deep-Sea Research 28:609-23.
- DOE (U.S. Department of Energy). 1994. Handbook of Methods for the Analysis of the Various Parameters of the Carbon Dioxide System in Seawater. Version 2.0. ORNL/CDIAC-74. Ed. A. G. Dickson and C. Goyet. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, Oak Ridge, Tenn.
- Friederich, G. E., L. A. Codispoti, and C. M. Sakamoto. 1991. An Easy-to-Construct Automated Winkler Titration System. Technical Report No. 91-6. Monterey Bay Aquarium Research Institute, Monterey, Calif.
- Friederich, G. E., P. Sherman, and L. A. Codispoti. 1984. A High-Precision Automated Winkler Titration System Based on a HP-85 Computer, A Simple Colorimeter and an Inexpensive Electromechanical Buret. Bigelow Laboratory Technical Report, 42. Bigelow Laboratory for Ocean Sciences, West Boothbay Harbor, Maine.
- Johnson, K. M. 1992. Operators Manual: Single-Operator Multiparameter Metabolic Analyzer (SOMMA) for Total Carbon Dioxide (CT) with Coulometric Detection. Brookhaven National Laboratory, Brookhaven, N.Y.
- Johnson, K. M., A. E. King, and J. McN. Sieburth. 1985. Coulometric TCO2 analyses for marine studies: An introduction. Marine Chemistry 16:61-82.
- Johnson, K. M., A. Kötzinger, L. Mintrop, J. C. Duinker, and D. W. R. Wallace. 1999. Coulometric total carbon dioxide analysis for marine studies: Measurement and internal consistency of underway surface TCO2 concentrations. Marine Chemistry 67:123-44.
- Johnson, K. M., P. J. Williams, L. Brandstrom, and J. McN. Sieburth. 1987. Coulometric total carbon analysis for marine studies: Automation and calibration. Marine Chemistry 21:117-33.
- Johnson, K. M., K. D. Wills, D. B. Butler, W. K. Johnson, and C. S. Wong. 1993. Coulometric total carbon dioxide analysis for marine studies: Maximizing the performance of an automated gas extraction system and coulometric detector. Marine Chemistry 44:167-87.
- Millero, F. J., A. G. Dickson, G. Eischeid, C. Goyet, P. R. Guenther, K. M. Johnson, K. Lee, E. Lewis, D. Purkerson, C. L. Sabine, R. Key, R. G. Schottle, D. R. W. Wallace, and C. D. Winn. 1998. Assessment of the quality of the shipboard measurements of total alkalinity on the WOCE Hydrographic Program Indian Ocean CO2 survey cruises, 19941996. Marine Chemistry 63:9-20.
- Millero F. J., J. Z. Zhang, K. Lee, and D. M. Campbell. 1993. Titration alkalinity of seawater. Marine Chemistry 44:153-65.
- Millero, F. J., X. Zhu, X. Liu, M. P. Roche, C. Moore, and J. Jolliff. 2000. The pH and TALK along 24N in the Atlantic Ocean. University of Miami Technical Report No. RSMAS-2000-03. University of Miami, Miami, Fla.
- Peng, T.-H., T. Takahashi, W. S. Broecker, and J. Olafsson. 1987. Seasonal variability of carbon dioxide, nutrients and oxygen in the northern North Atlantic surface water: Observations and a model. Tellus 39B:439-58.
- Wanninkhof, R., and K. Thoning. 1993. Measurement of fugacity of CO2 in surface water using continuous and discrete sampling methods. Marine Chemistry 44:189-205.
- Wilke, R. J., D. W. R. Wallace, and K. M. Johnson. 1993. A water-based, gravimetric method for the determination of gas sample loop volume. Analytical Chemistry 65:2403-06.
- Williams, P. J. LeB. Williams, and N. W. Jenkinson. 1982. A transportable microprocessor-controlled precise Winkler titration suitable for field station and shipboard use. Limnology and Oceanography 27:576-84.
- Zhang, J-Z., and G. A. Berberian. 1997. Determination of dissolved silicate in estuarine and coastal waters by gas segmented continuous flow colorimetric analysis. Methods for the Determination of Chemical Substances in Marine and Estuarine Environmental Matrices. 2nd ed. EPA/600/R-97/072. National Exposure Research Laboratory, Office of Research and Development, U.S. Environmental Protection Agency, Cincinnati, Ohio.
- Zhang, J-Z., C. Fischer, and P. B. Ortner. 2000. Comparison of open tubular cadmium reactor and packed cadmium column in automated gas-segmented continuous flow nitrate analysis. International Journal of Environmental Analytical Chemistry 76(2):99-113.
- Zhang, J-Z., C. Fischer, and P. B. Ortner. 2001. Continuous flow analysis of phosphate in natural waters using hydrazine as a reductant. International Journal of Environmental Analytical Chemistry 80(1):61-73.
- Zhang, J-Z., P. B. Ortner and C. Fischer. 1997. Determination of nitrite and nitrate in estuarine and coastal waters by gas segmented continuous flow colorimetric analysis. Methods for the Determination of Chemical Substances in Marine and Estuarine Environmental Matrices. 2nd ed. EPA/600/R-97/072. National Exposure Research Laboratory, Office of Research and Development, U.S. Environmental Protection Agency, Cincinnati, Ohio.
